🧪 Drug
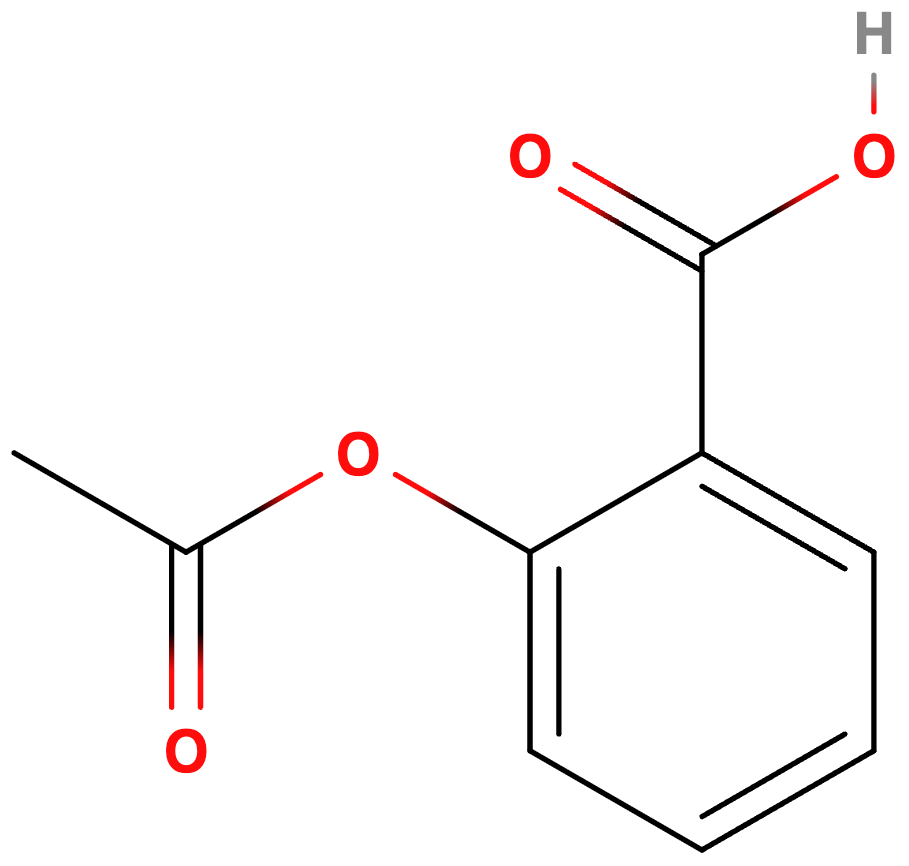
🩺 Aspirin - The Acidic Analgesic
Chemical Formula: C₉H₈O₄ (Acetylsalicylic Acid)
pKa: 3.5 - This low pKa means aspirin readily gives up protons in basic conditions
Mechanism: Irreversibly inhibits COX enzymes, reducing prostaglandin synthesis
Absorption Key: As a weak acid (pKa 3.5), aspirin stays mostly unionized in the acidic stomach (pH 1.5), allowing some absorption across cell membranes and some to undergo hydolysis. Most aspirin absorption happens in the small intestine, where the much larger surface area (200+ m²) overcomes the increased ionization that occurs at higher pH levels.
Clinical Insight: This is why aspirin can cause stomach irritation - it's absorbed right where it can damage the gastric lining!
🌡️ Environment
Aspirin
Aspirin (acetylsalicylic acid) - weak acid with pKa 3.5
📊 Model
Aspirin Henderson-Hasselbalch model (pKa = 3.5)